OSI Technology
Innovation in Oral and Nasal Biotechnologies
Oral Science International is an innovation driven emerging company, affiliated with Oral Science Canada. We are dedicated to developing and commercializing innovative oral and nasal bio-technologies and devices, providing solutions for respiratory infections and inflammation. Our technologies are licensed to the most capable distributors to bring them to market for you.
Actively identify and bring to market safe and efficacious therapeutics, treatments, and technologies to diagnose, treat, and protect society against viral and bacterial illness.
Mission
Actively identify and bring to market safe and efficacious therapeutics, treatments, and technologies to diagnose, treat, and protect society against viral and bacterial illness.
We are guided by a passion to discover and learn thought ongoing research. Our diverse team thrives with an open collaborative approach, guided by the benefit to the end user and customer. We embrace ideas across cultures and geographies for efficiency and to maximize the benefit our products bring to society.
Culture
We are guided by a passion to discover and learn thought ongoing research. Our diverse team thrives with an open collaborative approach, guided by the benefit to the end user and customer. We embrace ideas across cultures and geographies for efficiency and to maximize the benefit our products bring to society.
Our Goal is to Break the chain that leads to respiratory infection
200
Virus Strains
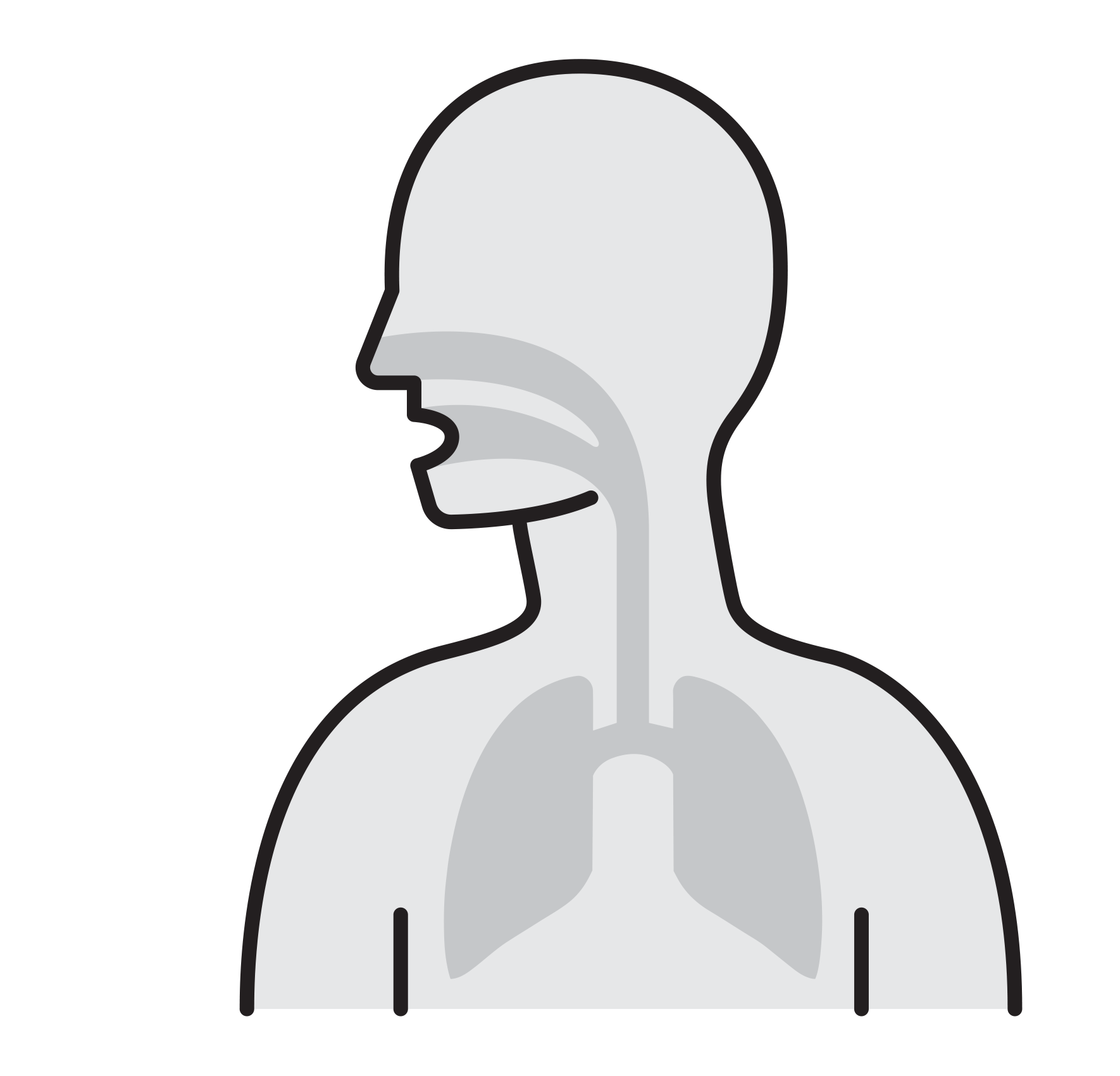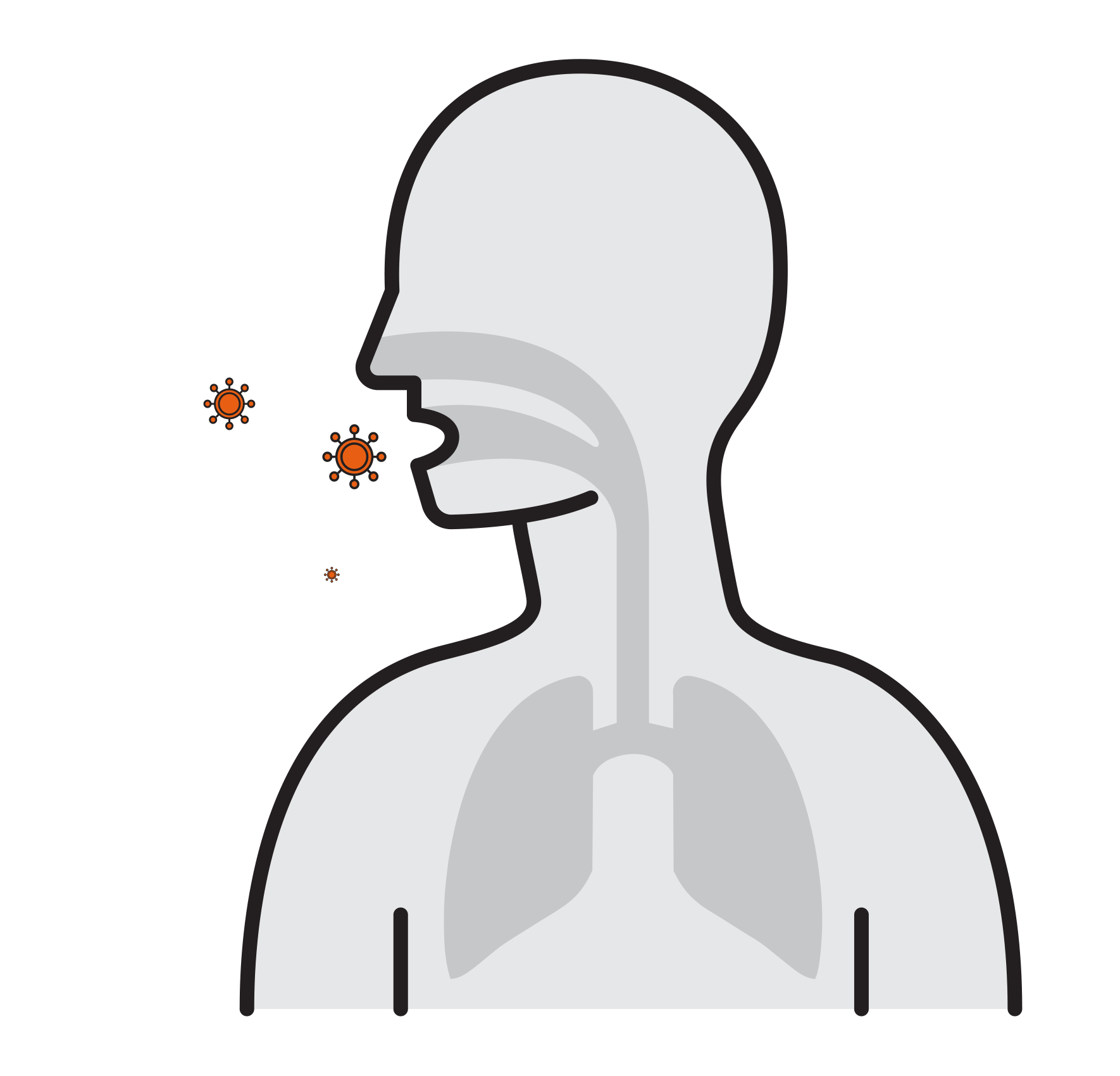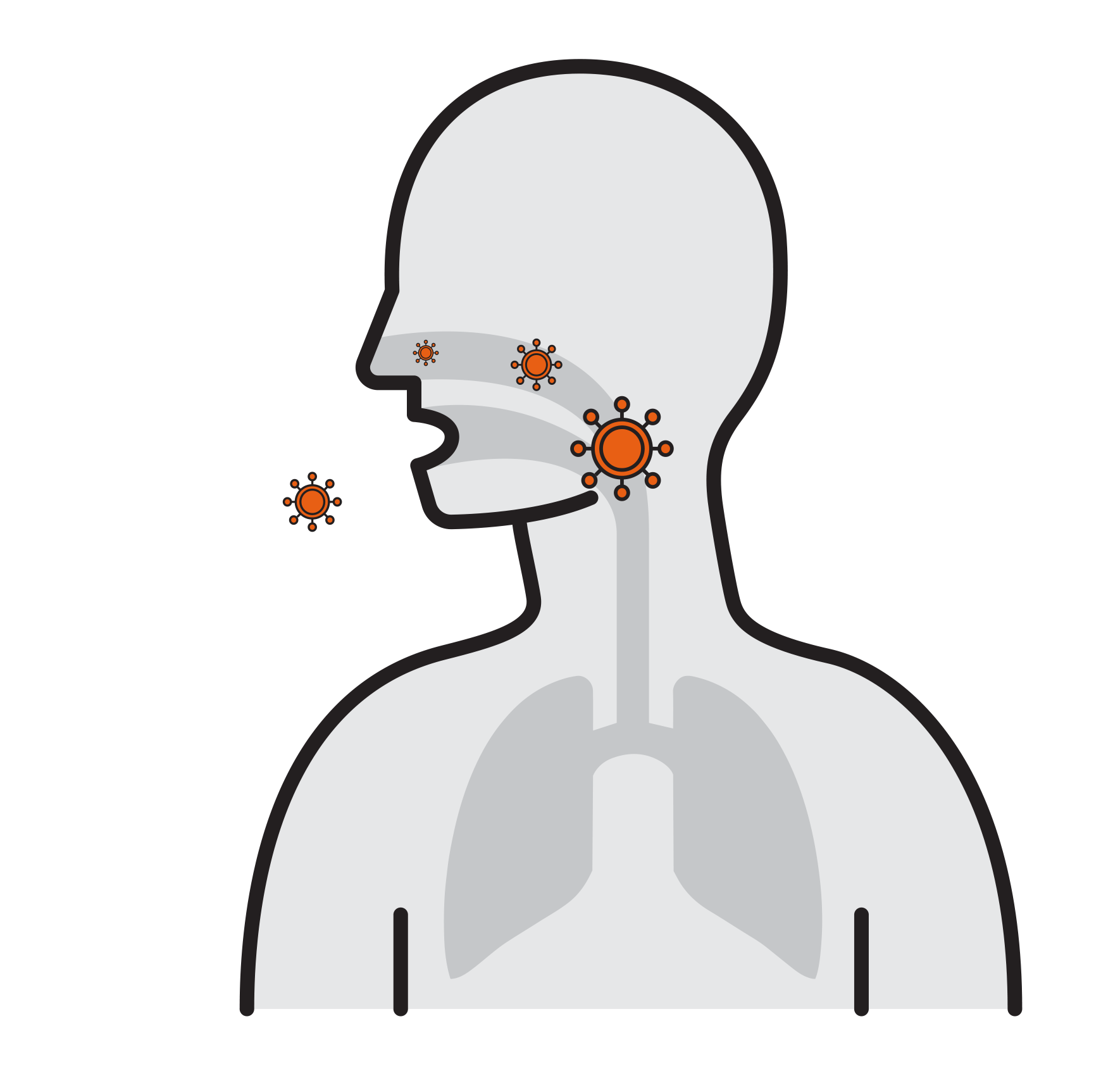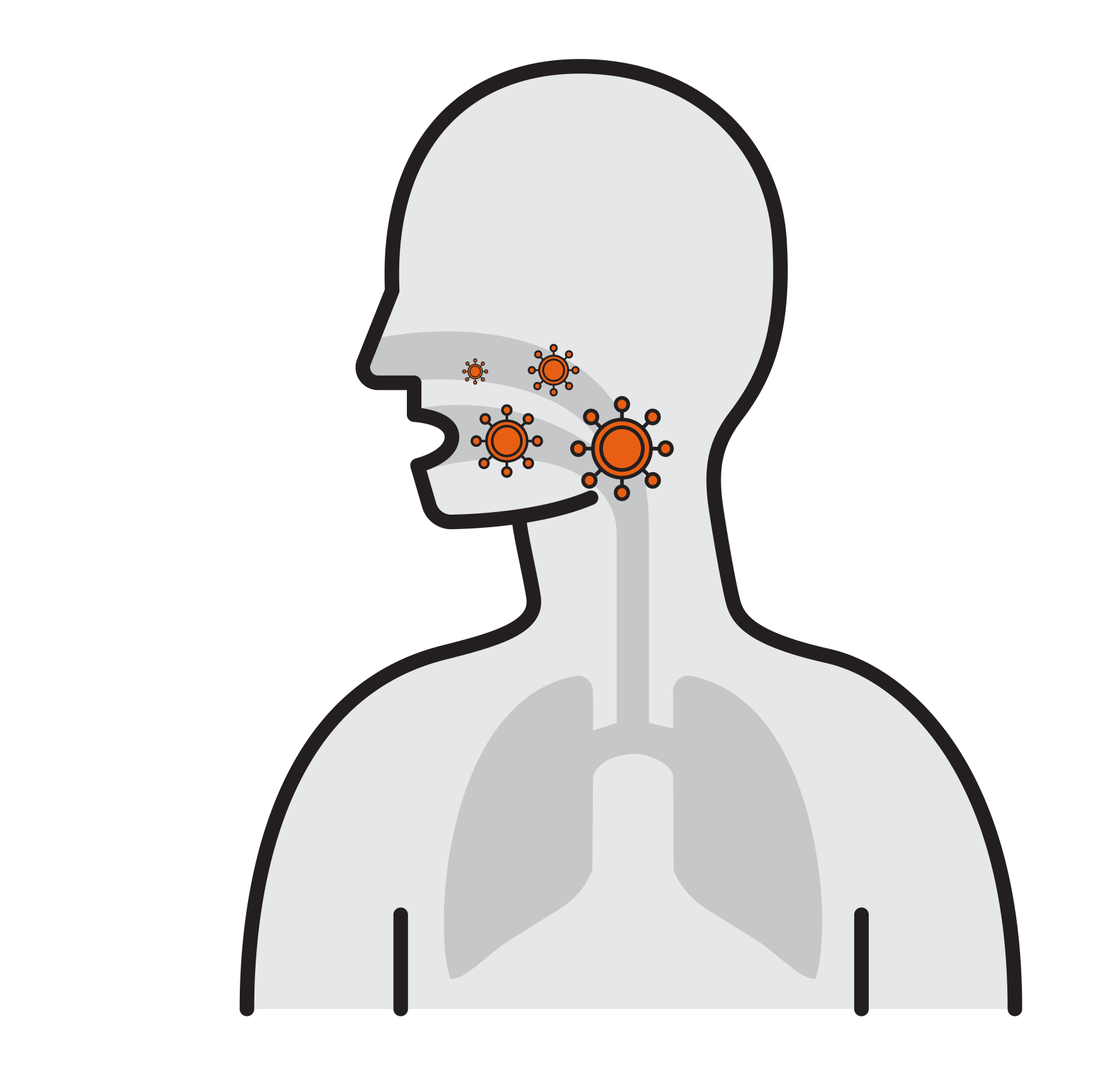
- Often spread by aerosols (ex. sneezing),
remain airborne for long time - Enter respiratory tract via the nose
and mouth - Contact large mucosa
Thousands are reproduced
in 8 hours
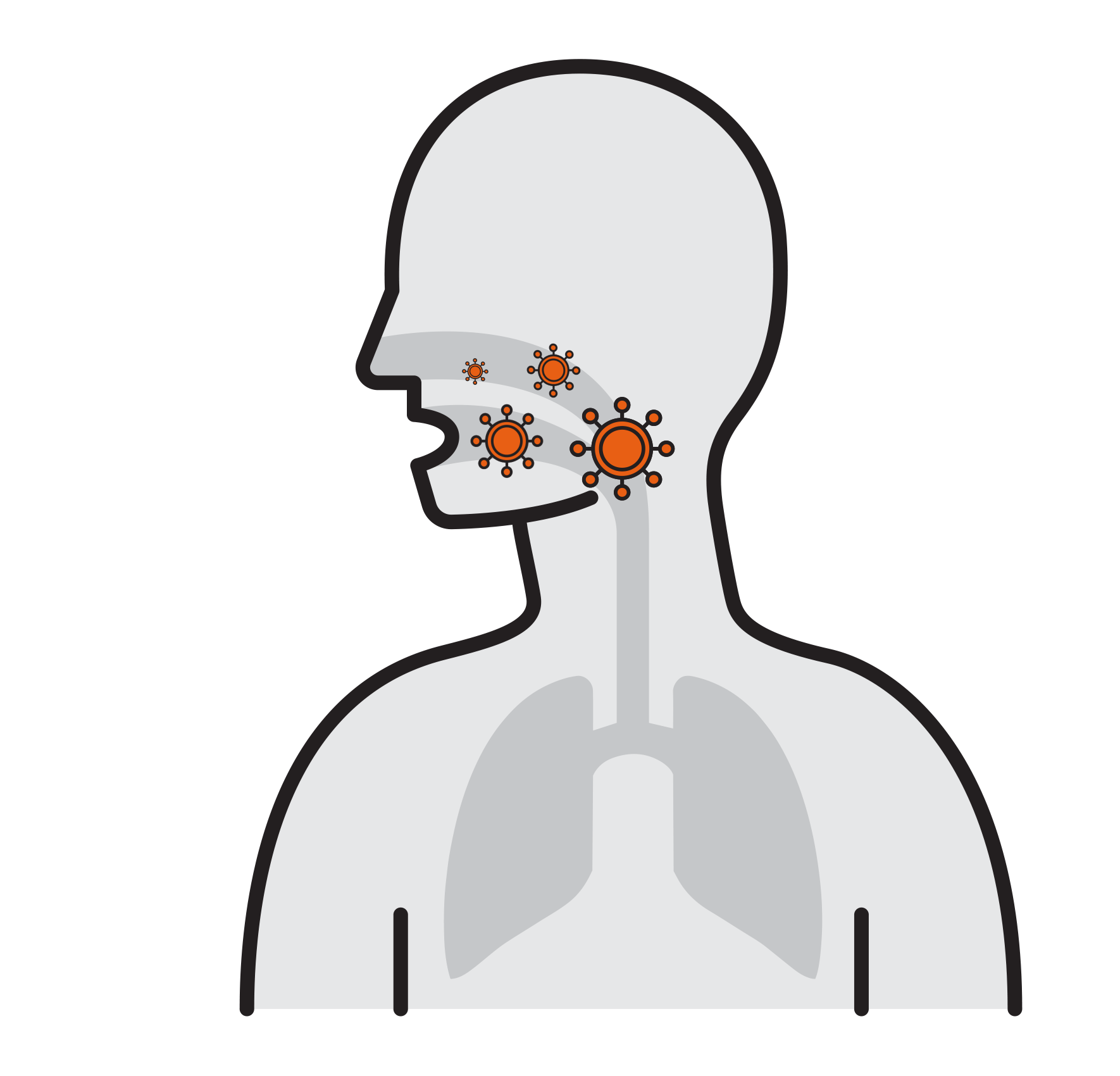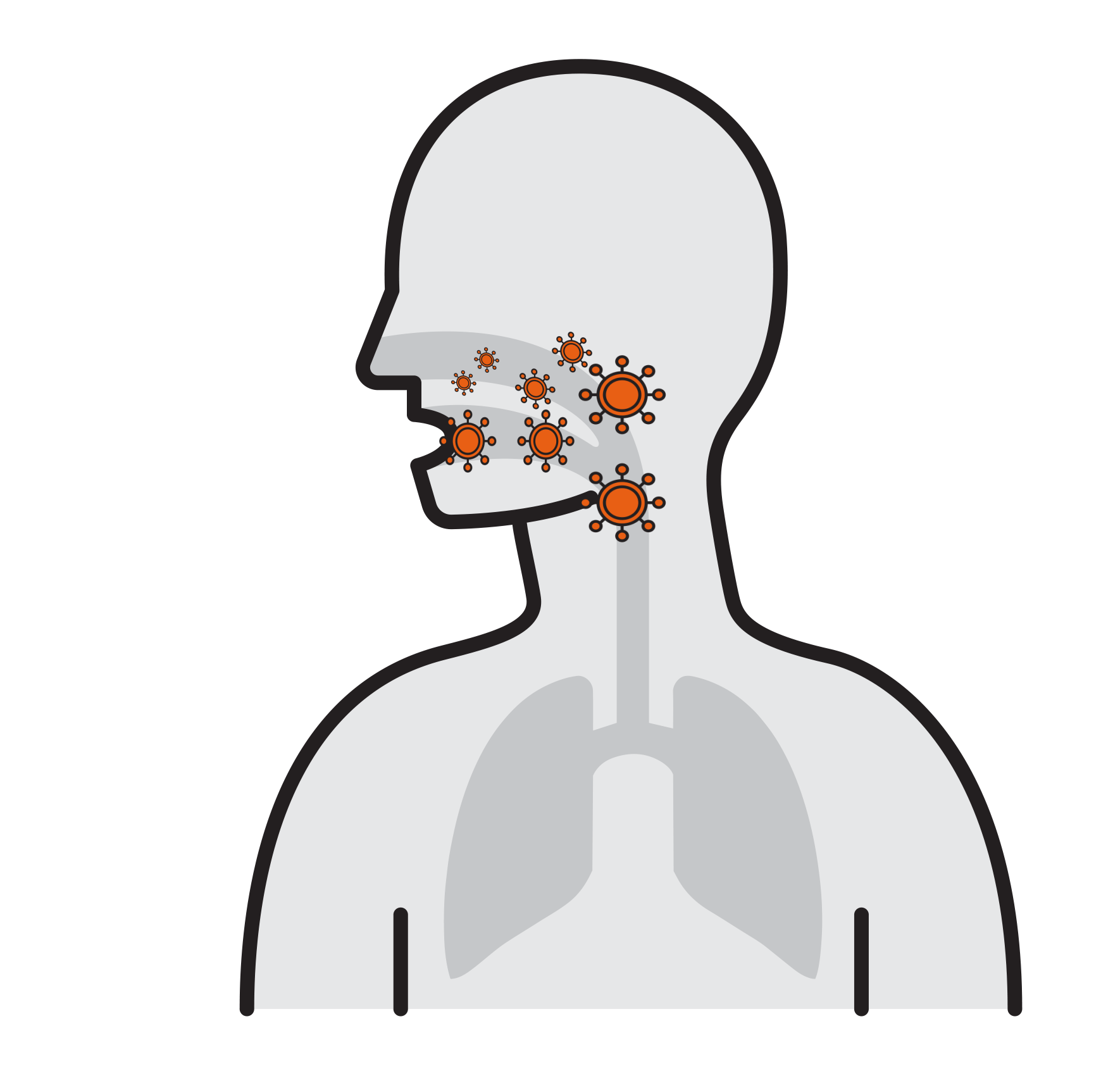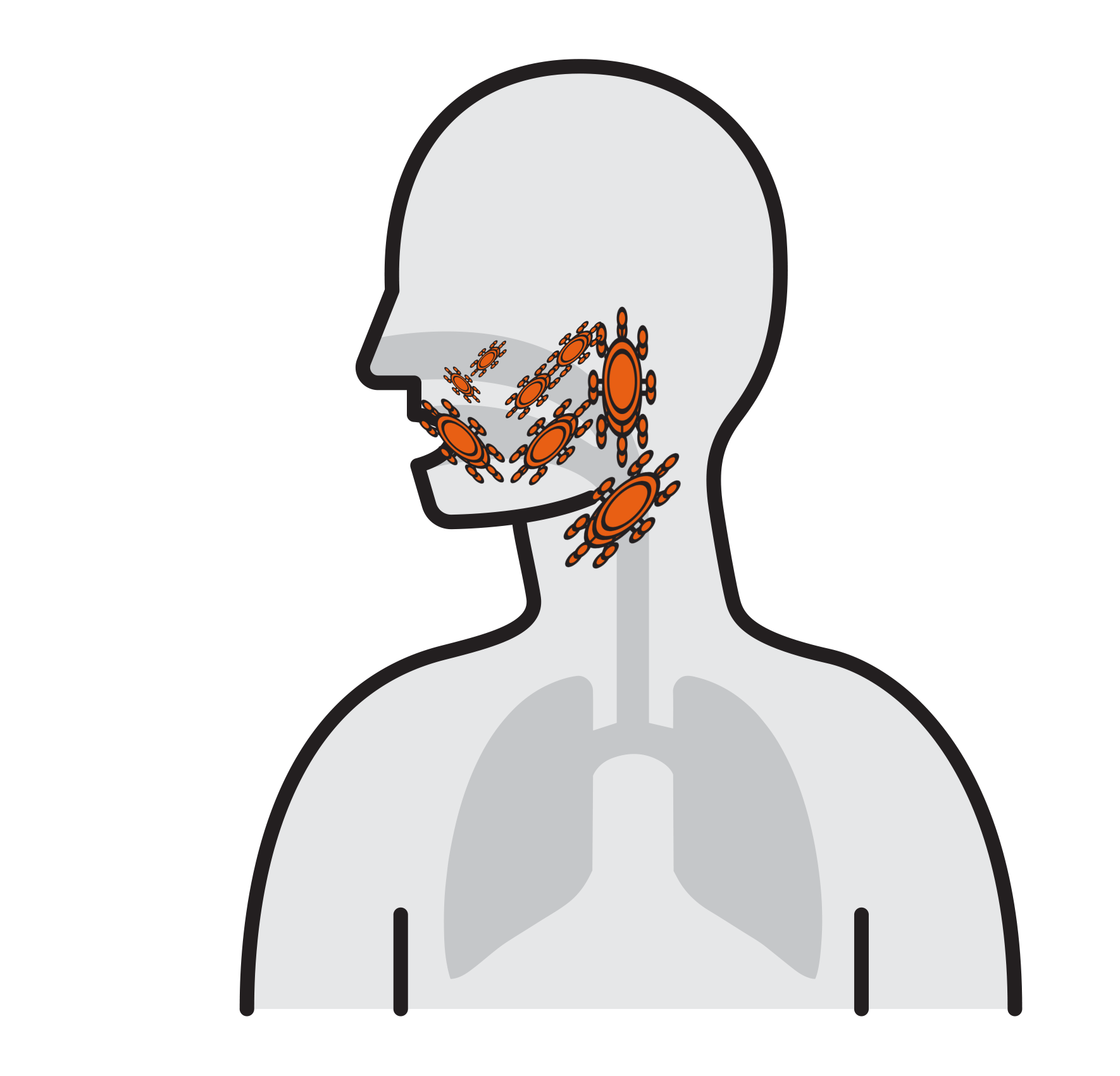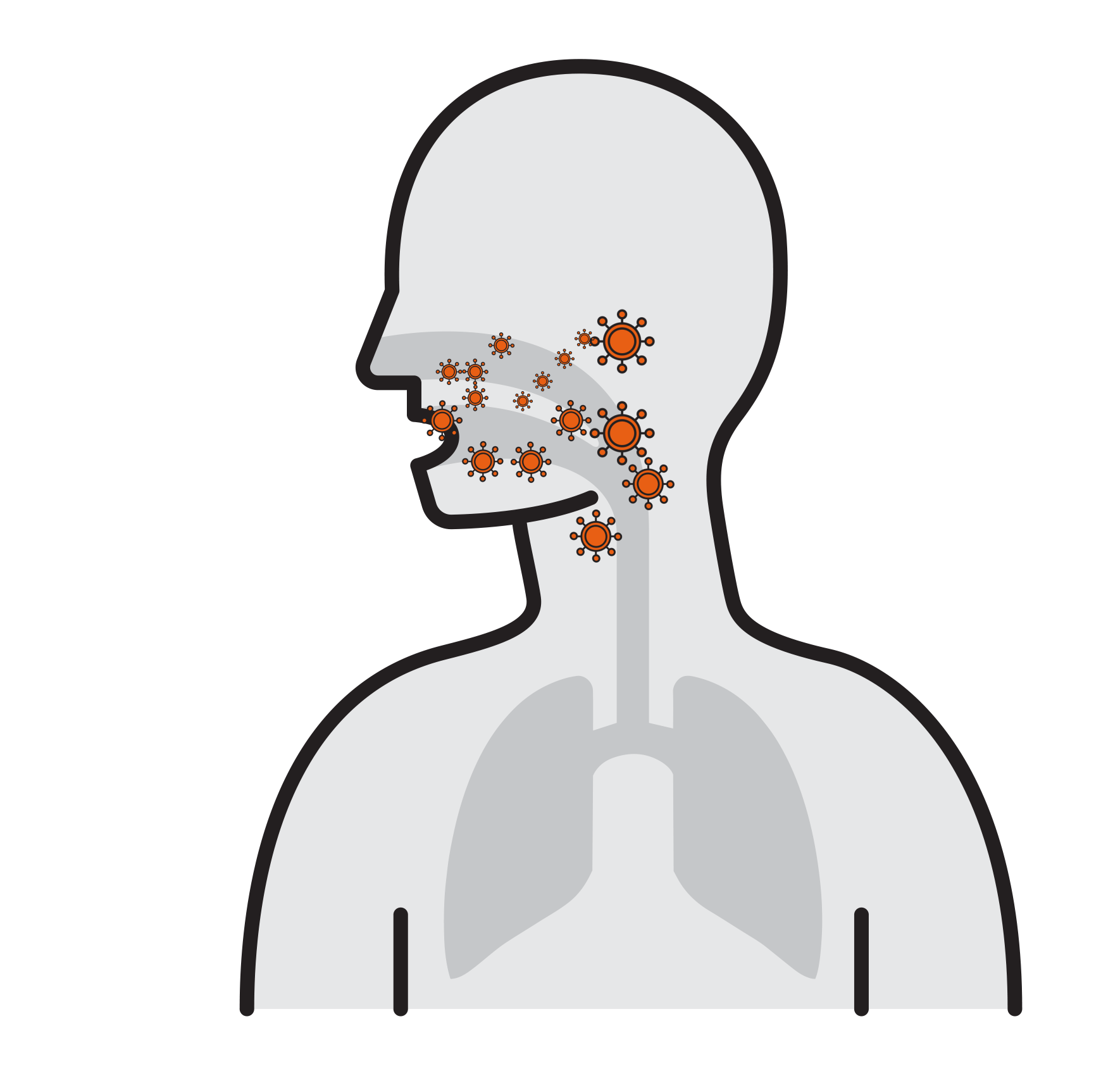
- Viruses attach to mucosa surfaces
- Virus is internalized to cells
- Reproduce
- Released and cause infection
Break
the Chain
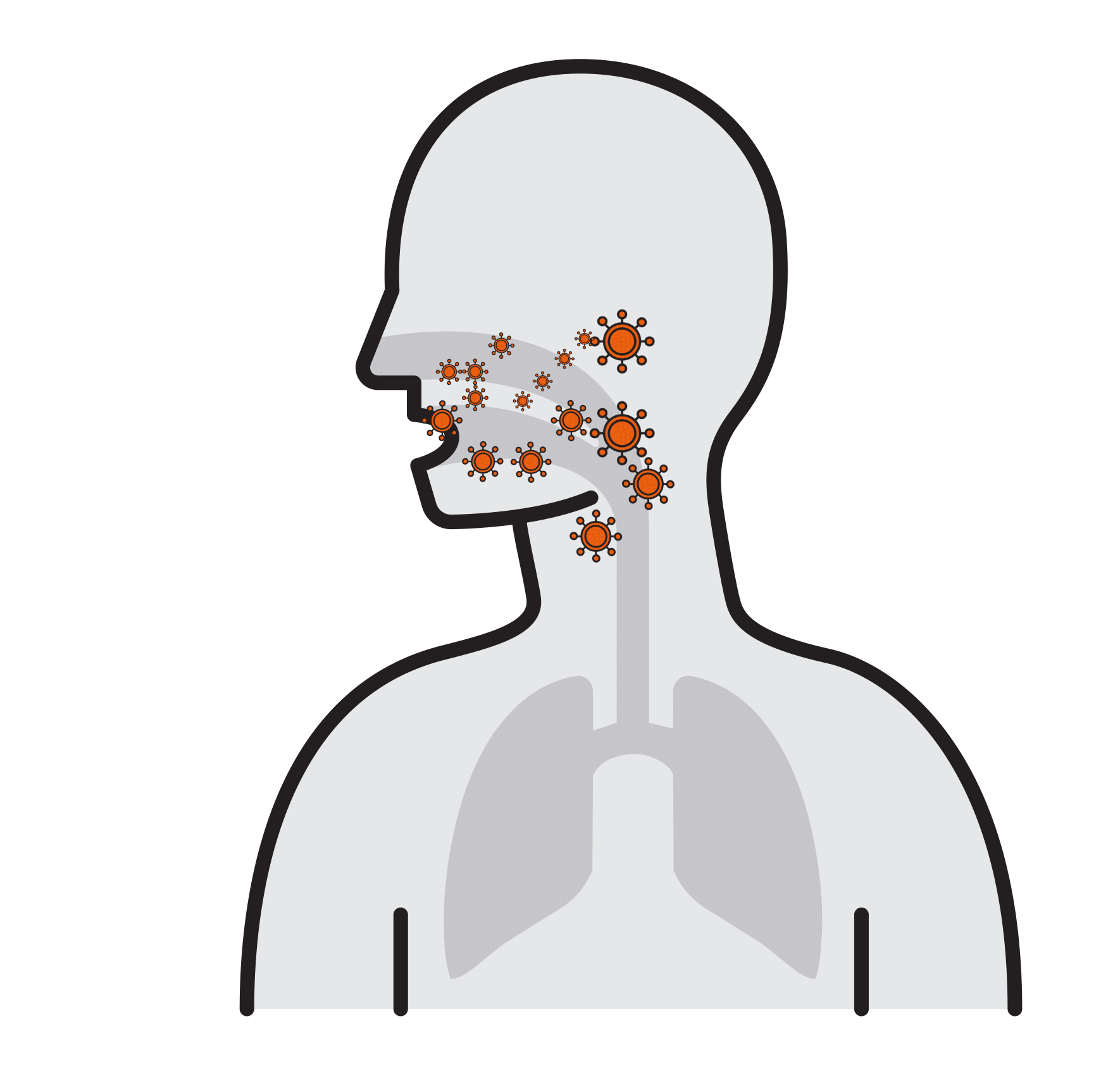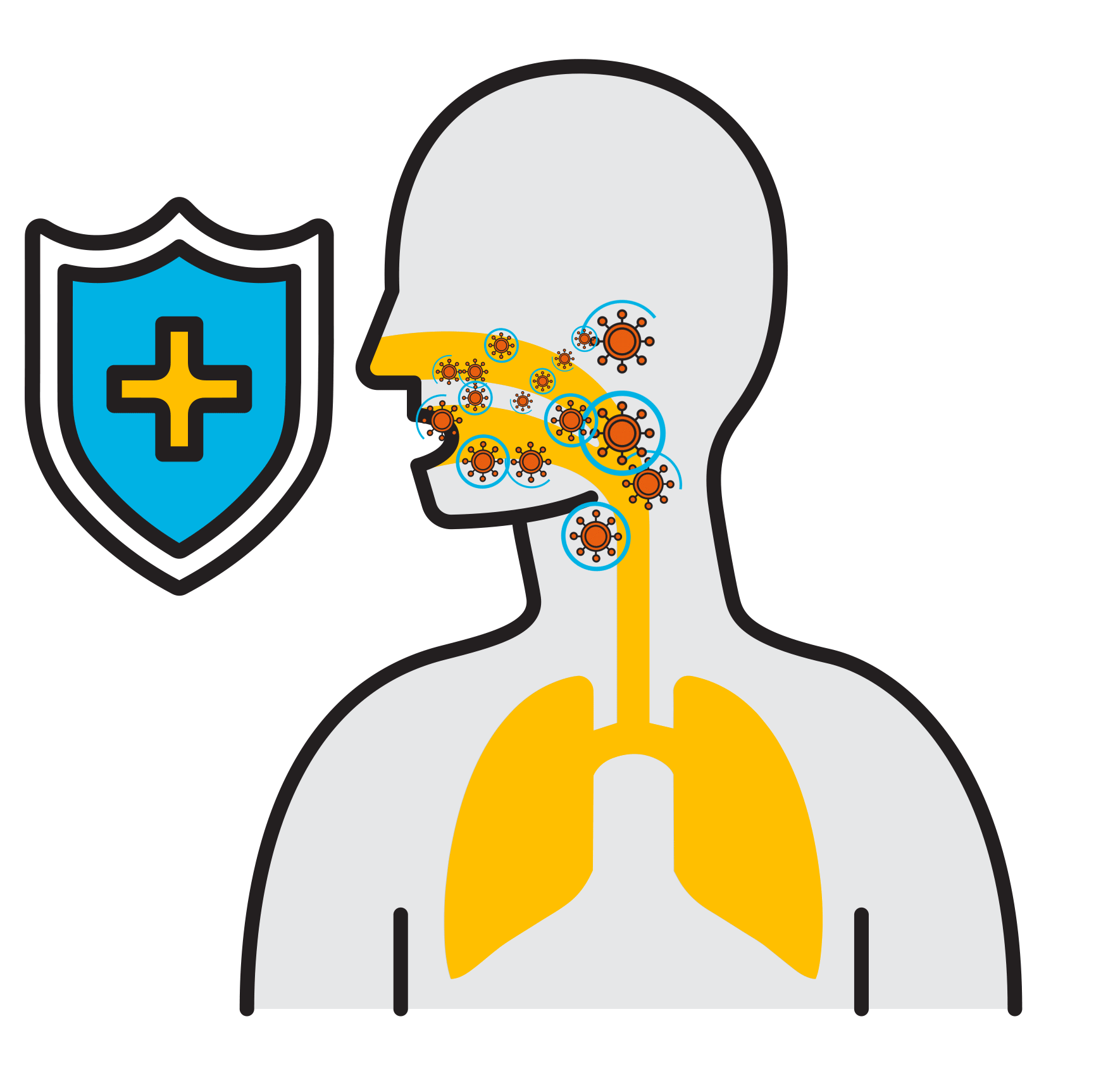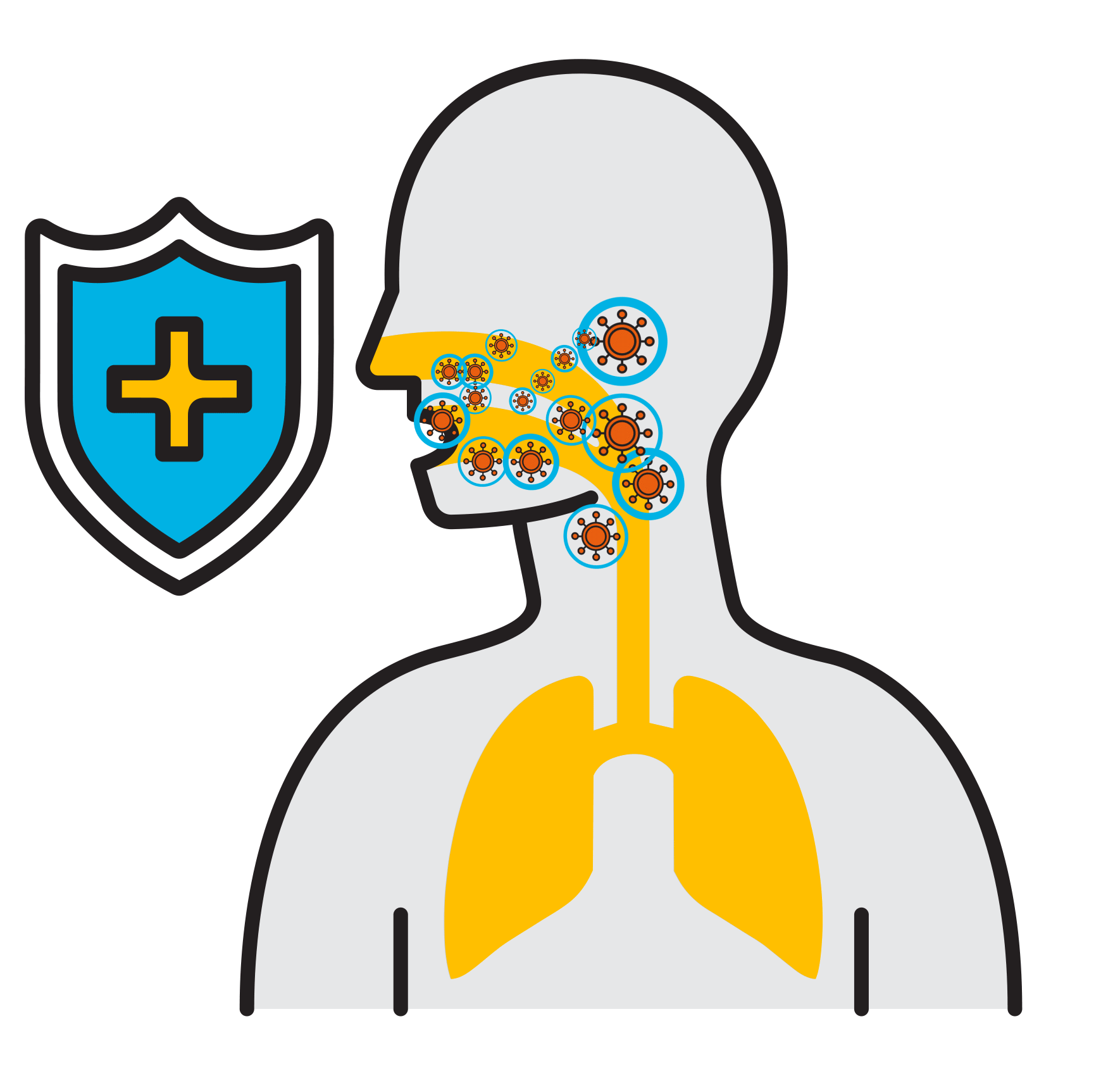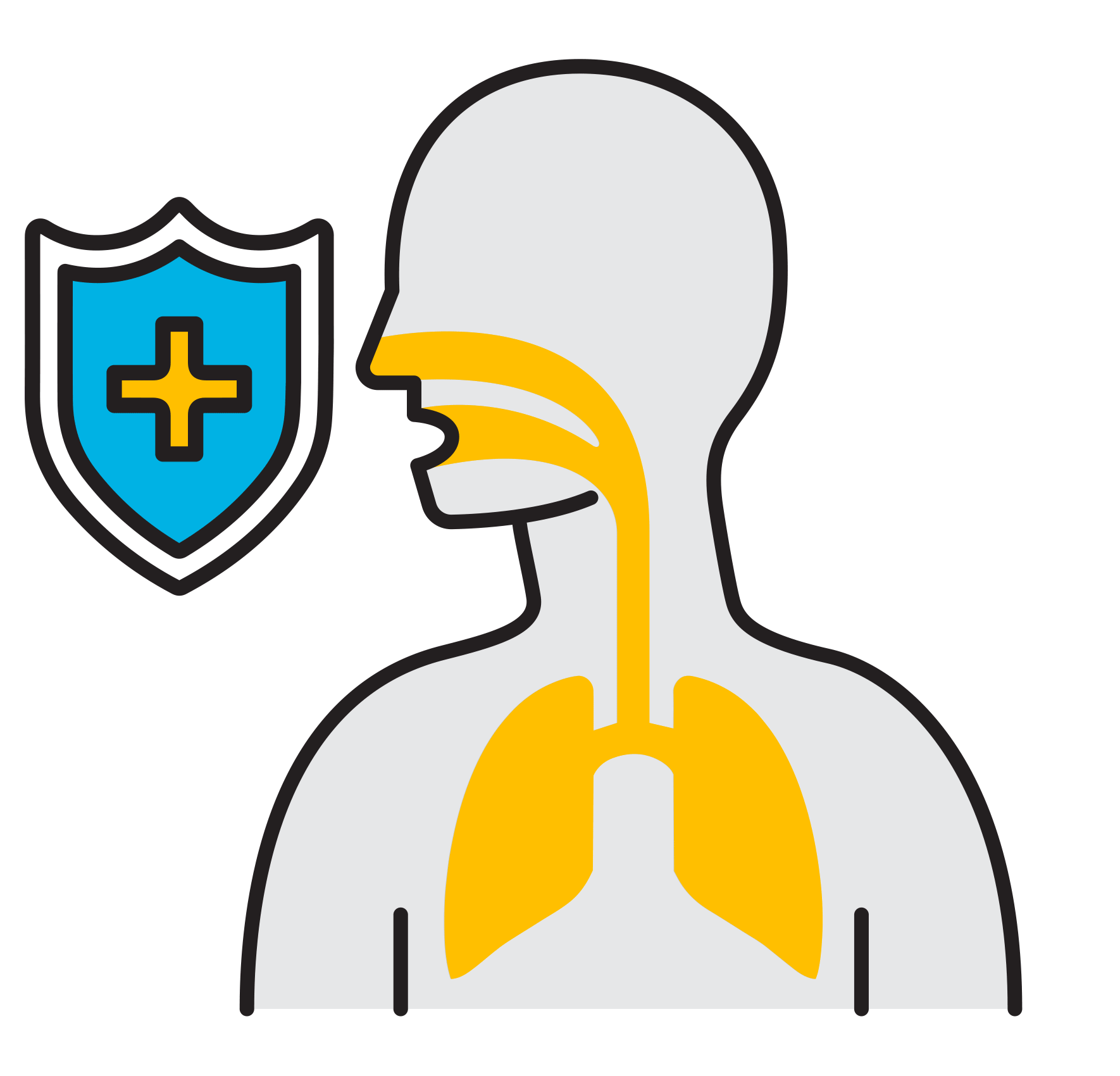
- Prevalent respiratory infections:
H1N1, H5N1, Influenza A, SARS-CoV,
Human Rhinoviruses.

Select Premium Ingredients
High Molecular Weight Hyaluronic Acid
- With over 14,000 studies in the past 10 years, is known for its effect on tissues, dermatology, inflammation, lubrication, its ability to bond water to tissue, and more
- Increases viscosity to ensure an efficient application to the throat and mouth

Flavobac™
- Patented, plant-based, specific complex of bioflavonoids
- Natural and organic
- Preserves and protects against viral activity
- Individually shown to destroy viruses’ envelopes (<1 min), deactivating their ability to further infect and multiply
- Higher antiviral activity than antiseptics

Glycerine
- A demulcent (derived from the Latin: demulcere “caress”) to form a soothing film over a mucous membrane, in this case your mouth and throat, relieving minor pain and inflammation

Menthol
- Anesthetic and analgesic
- For relief of pain and irritation
Studies and Articles on our Portfolio
Flavobac™
Scroll sideways to see full chart
STUDY NAME | BACTERIA / VIRUS TESTED AGAINST PRODUCT | PRODUCT EFFICACY AGAINST BACTERIA / VIRUS | TIME BACTERIA / VIRUS IS EXPOSED TO PRODUCT |
|---|---|---|---|
UTAH State University Virucidal Assay against SARS-CoV-2, Institute for Antiviral Research, SARS2-378. January 4 2021 | SARS-COV-2 | 98.741% | 1 minute |
Quantitative suspension test for evaluation of virucidal activity in the medical area (Phase 2 Step1), BS EN 14476, J002058-1, Microbiological Solutions Limited (MSL), July 22 2020 | Modified vaccinia virus Ankara (MVA), for enveloped viruses | 99.990% | 5 minutes |
Utah State University, Institute for Antiviral Research, Virucidal Assay against 9 Respiratory Viruses, for OSI 2011004-2, March 8 2021 | Influenza H1N1 H3N2 H5N1 ParaInfluenza PIV-3 Coronavirus hCOV-229E Rhinovirus HRV-16 Respiratory Syncytial Virus (RSV) Adenovirus-5 | 99.984% 98.415% 99.749% 94.988% 90.000% 99.900% 92.1% 0% | 1 minute 1 minute 1 minute 1 minute 1 minute 1 minute 1 minute 1 minute |
Utah State University, Institute for Antiviral Research, Virucidal Assay against SARS-COV-2 for OSIXX MARCH IN REVIEW | SARS-COV-2 | 98.741% | 5 minutes |
Cold & Flu Guard™
Scroll sideways to see full chart
STUDY NAME | BACTERIA / VIRUS TESTED AGAINST PRODUCT | PRODUCT EFFICACY AGAINST BACTERIA / VIRUS | TIME BACTERIA / VIRUS IS EXPOSED TO PRODUCT |
|---|---|---|---|
Quantitative suspension test for evaluation of virucidal activity in the medical area (Phase 2 Step1), BS EN 14476 J002058-3. Microbiological Solutions Limited (MSL), July 22 2020 | Modified vaccinia virus Ankara (MVA), for enveloped viruses | 99.944% | 5 minutes |
Utah State University, Institute for Antiviral Research, Virucidal Assay against 9 Respiratory Viruses, for OSI 2011004-2, March 8 2021 | Influenza H1N1 H3N2 H5N1 ParaInfluenza PIV-3 Coronavirus hCOV-229E Rhinovirus HRV-16 Rhinovirus HRV-14 Respiratory Syncytial Virus (RSV) Adenovirus-5 | 99.984% 98.415% 99.749% 94.988% 90.000% 99.990% 99.975% 92.1% 0% | 5 minutes 5 minutes 5 minutes 5 minutes 5 minutes 5 minutes 5 minutes 5 minutes 5 minutes |
Utah State University, Institute for Antiviral Research, Virucidal Assay against SARS-COV-2 for OSIXX MARCH IN REVIEW | SARS-COV-2 | 99.900% | 5 minutes |
- Lu, C.-W.; Liu, X.-F.; Jia, Z.-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet Lond. Engl. 2020, 395, e39.
- To, K.K.-W.; Tsang, O.T.-Y.; Chik-Yan Yip, C.; Chan, K.-H.; Wu, T.-C.; Chan, J.M.C.; Leung, W.-S.; Chik, T.S.-H.; Choi, C.Y.-C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. doi:org/10.1093/cid/ciaa149
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. T ransmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9.
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARSCoV- 2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177-1179.
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A Lesson in coexistence. PLoS Pathog. 2018, 14, e1006719.
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J.F. Acquiring and maintaining a normal oral microbiome: current perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85.
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 122–128.
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321.
- Lamarre, A.; Talbot, P.J. Effect of pH and temperature on the infectivity of human coronavirus 229E. Can. J. Microbiol. 1989, 35, 972–974.
- Geller, C.; Varbanov, M.; Duval, R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012, 4, 3044–3068.
- Tonoyan, L.; Vincent-Bugnas, S.; Olivieri, C.-V.; Doglio, A. New Viral Facets in Oral Diseases: The EBV Paradox. Int. J. Mol. Sci. 2019, 20, 5861.
- Giacaman, R.A.; Asrani, A.C.; Gebhard, K.H.; Dietrich, E.A.; Vacharaksa, A.; Ross, K.F.; Herzberg, M.C. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology 2008, 5, 29.
- M. F. Mahomoodally, A. Gurib-Fakim, and A. H. Subratty, Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharmaceutical Biology, vol. 43, no. 3, pp. 237–242, 2005.
- A. K. Pandey, Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Pariheniumhisterophorus: an in vitro study. National Academy Science Letters, vol. 30, no. 11-12, pp. 383–386, 2007.
- R. A. Dixon, P. M. Dey, and C. J. Lamb, Phytoalexins: enzymology and molecular biology. Advances in Enzymology and Related Areas of Molecular Biology, vol. 55, pp. 1–136, 1983.
- E. H. Kelly, R. T. Anthony, and J. B.Dennis, Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry, vol. 13, no. 10, pp. 572–584, 2002.
- S. Kumar, A. Mishra, and A. K. Pandey, Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complementary and Alternative Medicine, vol. 13, article 120, 2013.
- S. Kumar and A. K. Pandey, Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos, vol. 26, pp. 301–307, 2013.
- M. Leopoldini, N. Russo, S. Chiodo, and M. Toscano, Iron chelation by the powerful antioxidant flavonoid quercetin. Journal of Agricultural and Food Chemistry, vol. 54, no. 17, pp. 6343–6351, 2006.
- S. Kumar, A. Gupta, and A. K. Pandey, Calotropis procera root extract has capability to combat free radical mediated damage. ISRN Pharmacology, vol. 2013,Article ID 691372, 8 pages, 2013.
- N. C. Cook and S. Samman, Review: flavonoids-chemistry, metabolism, cardioprotective effects and dietary sources. Journal of Nutritional Biochemistry, vol. 7, no. 2, pp. 66–76, 1996. 12 The ScientificWorld Journal
- C. A. Rice-Evans, N. J. Miller, P. G. Bolwell, P. M. Broamley, and J. B. Pridham, The relative antioxidant activities of plantderived polyphenolic flavonoids. Free Radical Research, vol. 22, no. 4, pp. 375–383, 1995.
- G. Agati, E. Azzarello, S. Pollastri, and M. Tattini, Flavonoids as antioxidants in plants: location and functional significance Plant Science, vol. 196, pp. 67–76, 2012.
- F.Du, F. Zhang, F. Chen et al., Advances inmicrobial heterologous production of flavonoids. African Journal of Microbiology Research, vol. 5, no. 18, pp. 2566–2574, 2011.
- F. Pourmorad, S. J.Hosseinimehr, and N. Shahabimajd, Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. The African Journal of Biotechnology, vol. 5, no. 11, pp. 1142–1145, 2006.
- S. Kumar and A. K. Pandey, Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. International Review of Biophysical Chemistry, vol. 3, no. 3, pp. 42–47, 2012.
- Hooper, S.J., et al., Antimicrobial activity of Flavobac ™ bioflavonoid preparations against oral microorganisms. 2011. 210(1): p. E22.
- Aqil, F., I. Ahmad, and Z.J.T.j.o.B. Mehmood, Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. 2006. 30(3): p. 177-183.
- Fernandes, M., et al., Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. 2014. 60: p. 39-44.
- Tait, S., et al., Antiviral activity of substituted homoisoflavonoids on enteroviruses. 2006. 72(3): p. 252-255.
- Sindt, C.W., et al., Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study. 2012. 6: p. 511.
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79-89.
- Kavasi RM, Berdiaki A, Spyridaki I, Corsini E, Tsatsakis A, Tzanakakis G, Nikitovic D. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 2017;101:128-138.
- Valachová K, Volpi N, Stern R, Soltes L. Hyaluronan in Medical Practice. Curr Med Chem. 2016;23:3607-3617.
- Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94:14S–16S.
- Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–512.
- Linden GJ, Herzberg MC; Working group 4 of joint EFP/AAP workshop. Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40 Suppl 14:S20–S23.
- Utah State University, Institute for AntiViral Research: Virucidal Assay against SARS-CoV-2, 2021
- Data on file.
- Lu, C.-W.; Liu, X.-F.; Jia, Z.-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet Lond. Engl. 2020, 395, e39.
- To, K.K.-W.; Tsang, O.T.-Y.; Chik-Yan Yip, C.; Chan, K.-H.; Wu, T.-C.; Chan, J.M.C.; Leung, W.-S.; Chik, T.S.-H.; Choi, C.Y.-C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020. doi:org/10.1093/cid/ciaa149
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. T ransmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9.
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARSCoV- 2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177-1179.
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A Lesson in coexistence. PLoS Pathog. 2018, 14, e1006719.
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J.F. Acquiring and maintaining a normal oral microbiome: current perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85.
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 122–128.
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321.
- Lamarre, A.; Talbot, P.J. Effect of pH and temperature on the infectivity of human coronavirus 229E. Can. J. Microbiol. 1989, 35, 972–974.
- Geller, C.; Varbanov, M.; Duval, R.E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012, 4, 3044–3068.
- Tonoyan, L.; Vincent-Bugnas, S.; Olivieri, C.-V.; Doglio, A. New Viral Facets in Oral Diseases: The EBV Paradox. Int. J. Mol. Sci. 2019, 20, 5861.
- Giacaman, R.A.; Asrani, A.C.; Gebhard, K.H.; Dietrich, E.A.; Vacharaksa, A.; Ross, K.F.; Herzberg, M.C. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology 2008, 5, 29.
- M. F. Mahomoodally, A. Gurib-Fakim, and A. H. Subratty, Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharmaceutical Biology, vol. 43, no. 3, pp. 237–242, 2005.
- A. K. Pandey, Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Pariheniumhisterophorus: an in vitro study. National Academy Science Letters, vol. 30, no. 11-12, pp. 383–386, 2007.
- R. A. Dixon, P. M. Dey, and C. J. Lamb, Phytoalexins: enzymology and molecular biology. Advances in Enzymology and Related Areas of Molecular Biology, vol. 55, pp. 1–136, 1983.
- E. H. Kelly, R. T. Anthony, and J. B.Dennis, Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry, vol. 13, no. 10, pp. 572–584, 2002.
- S. Kumar, A. Mishra, and A. K. Pandey, Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complementary and Alternative Medicine, vol. 13, article 120, 2013.
- S. Kumar and A. K. Pandey, Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts. Vegetos, vol. 26, pp. 301–307, 2013.
- M. Leopoldini, N. Russo, S. Chiodo, and M. Toscano, Iron chelation by the powerful antioxidant flavonoid quercetin. Journal of Agricultural and Food Chemistry, vol. 54, no. 17, pp. 6343–6351, 2006.
- S. Kumar, A. Gupta, and A. K. Pandey, Calotropis procera root extract has capability to combat free radical mediated damage. ISRN Pharmacology, vol. 2013,Article ID 691372, 8 pages, 2013.
- N. C. Cook and S. Samman, Review: flavonoids-chemistry, metabolism, cardioprotective effects and dietary sources. Journal of Nutritional Biochemistry, vol. 7, no. 2, pp. 66–76, 1996. 12 The ScientificWorld Journal
- C. A. Rice-Evans, N. J. Miller, P. G. Bolwell, P. M. Broamley, and J. B. Pridham, The relative antioxidant activities of plantderived polyphenolic flavonoids. Free Radical Research, vol. 22, no. 4, pp. 375–383, 1995.
- G. Agati, E. Azzarello, S. Pollastri, and M. Tattini, Flavonoids as antioxidants in plants: location and functional significance Plant Science, vol. 196, pp. 67–76, 2012.
- F.Du, F. Zhang, F. Chen et al., Advances inmicrobial heterologous production of flavonoids. African Journal of Microbiology Research, vol. 5, no. 18, pp. 2566–2574, 2011.
- F. Pourmorad, S. J.Hosseinimehr, and N. Shahabimajd, Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. The African Journal of Biotechnology, vol. 5, no. 11, pp. 1142–1145, 2006.
- S. Kumar and A. K. Pandey, Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. International Review of Biophysical Chemistry, vol. 3, no. 3, pp. 42–47, 2012.
- Hooper, S.J., et al., Antimicrobial activity of Flavobac ™ bioflavonoid preparations against oral microorganisms. 2011. 210(1): p. E22.
- Aqil, F., I. Ahmad, and Z.J.T.j.o.B. Mehmood, Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. 2006. 30(3): p. 177-183.
- Fernandes, M., et al., Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. 2014. 60: p. 39-44.
- Tait, S., et al., Antiviral activity of substituted homoisoflavonoids on enteroviruses. 2006. 72(3): p. 252-255.
- Sindt, C.W., et al., Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study. 2012. 6: p. 511.
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79-89.
- Kavasi RM, Berdiaki A, Spyridaki I, Corsini E, Tsatsakis A, Tzanakakis G, Nikitovic D. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 2017;101:128-138.
- Valachová K, Volpi N, Stern R, Soltes L. Hyaluronan in Medical Practice. Curr Med Chem. 2016;23:3607-3617.
- Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94:14S–16S.
- Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–512.
- Linden GJ, Herzberg MC; Working group 4 of joint EFP/AAP workshop. Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40 Suppl 14:S20–S23.
- Utah State University, Institute for AntiViral Research: Virucidal Assay against SARS-CoV-2, 2021
- Data on file.
OTHER STUDIES OF INTEREST
After conducting intensive laboratory tests on the effects on viruses and bacteria; in vitro results demonstrated that Flavobac™ is demonstrated effective neutralizing viruses and bacteria within 5 minutes.
VIRUSES
- Human Rhinoviruses (HRVs)
- SARS-coV (Urbani)
- Influenza A
- H1N1
- H3N2
- H5N1
- Herpes simplex virus type 1
- Herpes simplex virus type 2
- Herpes zoster
- Hepatitis A
- Hepatitis B
- Human Immunodeficiency Virus (HIV)
- African swine fever
- Coxsackievirus (Hand-foot-and-mouth disease)
- Gumboro disease
- Newcastle disease
PROTOZOA
- Histomonas meleagridis
- Giardia lamblia
- Entamoeba histolytica
- Blastocystis hominis
YEAST & FUNGI
- Aspergillus flavus
- Aspergillus niger
- Aspergillus terreus
- Botrytis cinerea
- Candida albicans
- Candida glabrata
- Chaetomium globosum
- Cladosporium
- Colletotrichum sp.
- Fusarium sp.
- Mucor sp.
- Penicillium sp.
- Penicillium digitatum
- Penicillium funiculosum
- Penicillium italicum
- Penicillium roqueforti
- Phomopsis portal
- Pullularia pullulans
- Pythium spp
- Trichophyton interdigitale
- Trichophyton mentagrophytes
BACTERIA
- A. viscosus
- Campylobacter jejuni
- Diplodia natalensis
- C. difficile
- Escherichia coli (E. coli)
- Geotrichum candidum
- Klebsiella pneumonia
- Lactobacillus Pentosus
- Legionella pneumophila (NCTC 11192)
- Listeria monocytogenes
- Mycobacterium fortuitum (NCTC 8573)
- Proteus vulgaris
- P. gingivalis
- P. intermedia
- Pseudomonas aeruginosa (ATCC 15442)
- Staphylococcus aureus (MRSA clinical strain)
- S. gordonii
- S. sanguinis
- T. denticola
*All of the pathogens/viruses are tested at independent laboratories. Certificates and reports available on request.




